Press releases



The report highlights the significant burden that mental health conditions place on European societies and healthcare systems.

Angelini Pharma today shared positive findings from its clinical trials in epilepsy.

Angelini Pharma will award 25,000 $, with the potential of further awards not less than $5,000.

Milestone coincides with expansion of cenobamate access to more than 100 markets worldwide

Eco-anxiety, characterized by a pervasive fear of climate change and its consequences, has emerged as a significant concern for mental health, particularly among young people.

The report shows up to 40% of European patients living with epilepsy are receiving inadequate treatment to control seizures.

LIFE-GREENAPI will deploy a more efficient, greener, and innovative process for producing Active Pharmaceutical Ingredients (APIs), by switching from batch to flow chemistry technology.

Angelini Pharma announces that the European Commission (EC) has approved the extension of the indication of Xydalba® (dalbavancin) for the treatment of acute bacterial skin and soft tissue infections in children.

Jacopo Andreose, who will take on the role from February 6, has over twenty years of experience in top management positions in the pharmaceutical industry.

Ukraine–Russia conflict and climate change may lead to a new wave of mental health disorders in the coming months.

In his position, Manarini will report hierarchically to the CEO, Pierluigi Antonelli and will join the Leadership Team-the executive committee-of Angelini Pharma.

The report highlights the need for closer European collaboration to tackle the burden of epilepsy

Rafal Kaminski has broad international experience in drug discovery and innovation and will lead Angelini Pharma’s research and development (R&D) strategy. He will commence his role in January 2022.

The COVID 19 pandemic has exacerbated gender-related challenges with its mental health consequences disproportionately affecting women, both at work and at home.

The fund has been established to invest in early-stage companies in Canada and U.S. markets, that are developing pharmaceutical therapies for central nervous system disorders (CNS) and rare diseases.

Angelini Pharma announced today that the EC has granted marketing authorization for ONTOZRY®.

Four thousand European citizens were interviewed by SWG on behalf of Angelini Pharma on the occasion of the Purple Day, the international day of awareness about epilepsy. According to the majority of respondents, people with epilepsy are perfectly normal people, but 40% would not talk about the disease at work.

Elma Research conducted three interviews with the presidents of three epilepsy-fighting organizations.

Through the acquisition, Angelini Pharma will be a major player in the market for treatment of central nervous system (CNS) and mental health disorders.

This acquisition will propel Angelini Pharma into a leading European player, well positioned to address the needs of patients with different CNS and mental disorders.

In his role, Ghirlanda reports directly to CEO Pierluigi Antonelli and joins the Executive Leadership Team, the Company's Steering Committee.

Social networks feature the events of the “Monelli family” to learn new health and hygiene habits in a fun, light-hearted manner. And on TikTok, rules become choreographies.

Promoting the development of corporate decision-making process powered by the use of data.

Enter into Exclusive License Agreement to Develop, Manufacture and Commercialize OV101 (gaboxadol) for the potential treatment of Angelman Syndrome in Europe.

In her role she will report to CEO Pierluigi Antonelli and will be part of the Executive Leadership Team, the Company's Steering Committee.

In her role she will report to CEO Pierluigi Antonelli and will continue to be part of the Executive Leadership Team, Angelini Pharma’s steering committee, which she joined in April 2019.

In his role, Kindling will be directly reporting to Luigi Cianci, Chief Commercial Officer – International.

La grande incertezza” is also a multi-channel communication campaign.

The 1 million euros donation will reinforce research laboratories where COVID-19 was isolated in Italy for the first time.

Angelini Pharma remains mobilized in the fight against the spread of COVID-19 to protect our doctors, nurses, medical workers on the front lines and all Italians. With Italy. For Italy.

Angelini Pharma announces today it has acquired the ThermaCare® global business rights, excluding North America, from GSK. The deal also includes the dedicated US manufacturing site for ThermaCare in Albany, Georgia.

Daniela Poggio is joining Angelini Pharma in the new role of Global Communications Head, reporting directly to CEO Pierluigi Antonelli.

With his many years of experience in roles of increasing responsibility in multinationals (Wyeth, Abbott, Bristol-Myers Squibb) as well as leading Italian companies (Chiesi), Fabio De Luca is now taking the helm of Global Marketing at Angelini Pharma, having successfully strengthened our Italian business in the Prescription Medication and Consumer Healthcare sectors.

Angelini Pharma announces the acquisition of two Sanofi CHC brands in Germany and Austria for a total of € 47 M.

Sumitomo Dainippon Pharma Co., Ltd. and Angelini S.p.A. announced today that the companies have formed a partnership with the goal to expand availability in Europe of Latuda®, an atypical antipsychotic.
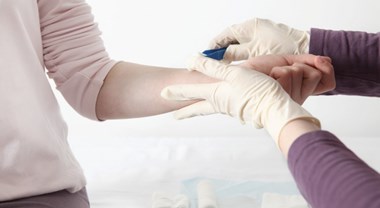
The new acid-oxidising solution containing hypochlorous acid and intended for cleansing various kind of wounds, including diabetic foot ulcers, pressure and vascular ulcers, has arrived in Italy.

Depression affects 7.5 million people, namely 12.5% of the population in Italy. According to the World Health Organisation (WHO), this illness is the first reason for disability nowadays.